Essential Petroleum Refining Processes and Origin Theories
Catalytic Reforming Process Fundamentals
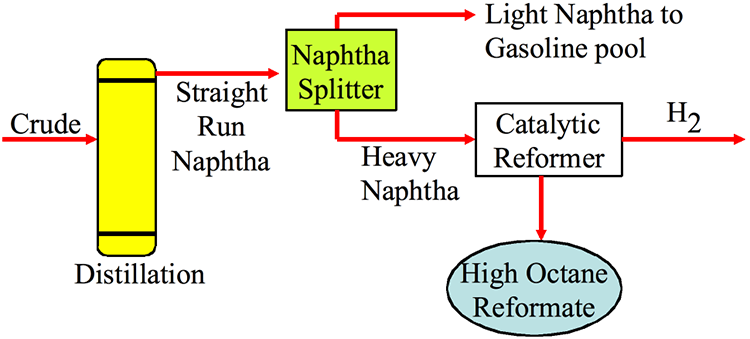
- Converts low-octane naphtha into high-octane reformate, which is used for gasoline blending and aromatics production.
- Feed is mixed with hydrogen, heated, and passed over a catalyst in fixed-bed reactors.
- Major reactions include dehydrogenation to aromatics, isomerization to branched paraffins, and mild hydrocracking.
- Operating conditions are typically 450–520 °C temperature and 10–45 atm pressure.
- The catalyst used is platinum on alumina or bimetallic Pt–Re on alumina.
- Main products are reformate, hydrogen, LPG, and light hydrocarbons.
Fluid Catalytic Cracking (FCC) Technology
- FCC converts heavy gas oils into valuable products like high-octane gasoline, LPG, and olefins.
- Preheated feed contacts hot, powdered zeolite catalyst in a riser reactor.
- Cracked vapors are separated from the catalyst in cyclones and sent to a fractionator.
- Spent catalyst is regenerated by burning off coke deposits and then recycled to the reactor.
- Operating conditions are typically 480–550 °C temperature and 1.5–3 atm pressure.
- The primary catalyst used is Y-type zeolite.
- Products obtained include high-octane gasoline, LPG, olefins, and light cycle oil.
- The flow diagram is summarized as: Riser → Cyclones → Fractionator → Products; Catalyst → Regenerator → Recycle.
Flexi Coking: Converting Heavy Feedstocks
- The petroleum industry, especially with advancements in gasification and petrochemicals, utilizes Flexi Coking, which integrates coking and gasification operations.
- This integration allows refineries to convert vacuum residuums and other heavy feedstocks into desulfurized liquids and gases.
- As a result, up to 99% of vacuum residuums are transformed into liquids or gases containing less than 1% sulfur.
- In the process, residuum feed is injected into a reactor where thermal cracking occurs, converting the heavy oil into lighter products and coke.
Hydrocarbon Formation Theories
- Scientists are still unable to fully and successfully explain the origin and formation of large hydrocarbon (crude oil) deposits; a sound theory is yet to be universally accepted.
Inorganic Theory (Mendeleev and Berthelot)
They tried to explain hydrocarbon deposits through inorganic reactions, especially focusing on the activity of the acetylene series.
Certain carbides, like calcium carbide (CaC2) and aluminum carbide (Al4C3), can react with water to form hydrocarbons. Examples of these reactions include:
- CaC2 + 2H2O → C2H2 + Ca(OH)2
- Al4C3 + 12H2O → 3C2H2 + 4Al(OH)3
- The theory assumed such carbides existed in the Earth's crust and could form large deposits of hydrocarbons, but this explanation could not account for the vast quantities found.

 English with a size of 3.35 KB
English with a size of 3.35 KB